Regenerative Medicineregenerative medicine
We invested at the founding of Tele Bio Inc., a certified venture originating from Jichi Medical University, and through joint research we provide research reagents related to regenerative medicine, including human adipose-derived stem cell secretome.
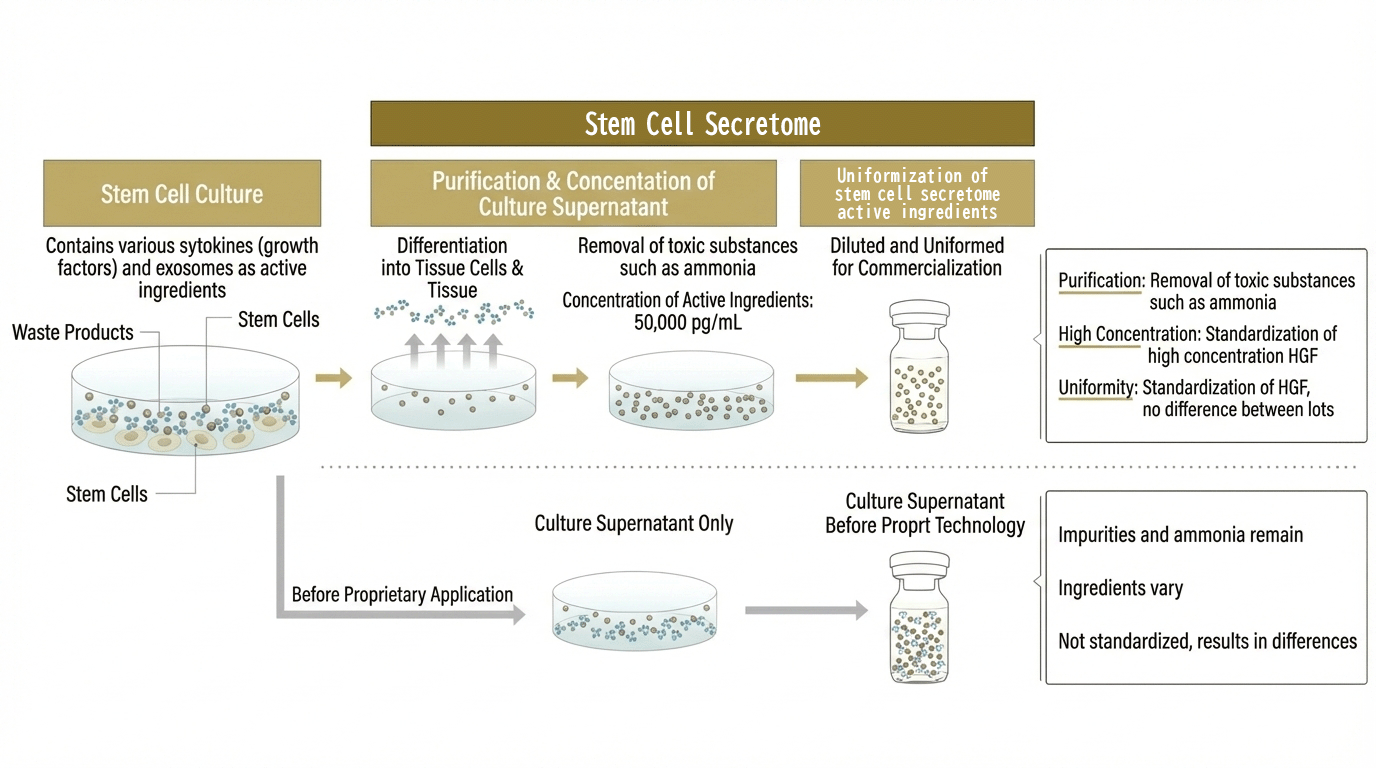
Key Features of the Stem Cell Secretome (Proprietary Technology)
- First in the world to develop stem cell secretome that is purified, concentrated, and standardized using proprietary technology.
- Removes impurities (such as ammonia and lactic acid) and increases the concentration of hepatocyte growth factor (HGF), which is considered important for tissue regeneration.
Three Potential Benefits of the Stem Cell Secretome
- 1) Tissue repair and protection
- 2) Anti-inflammatory effects
- 3) Anti-fibrotic effects
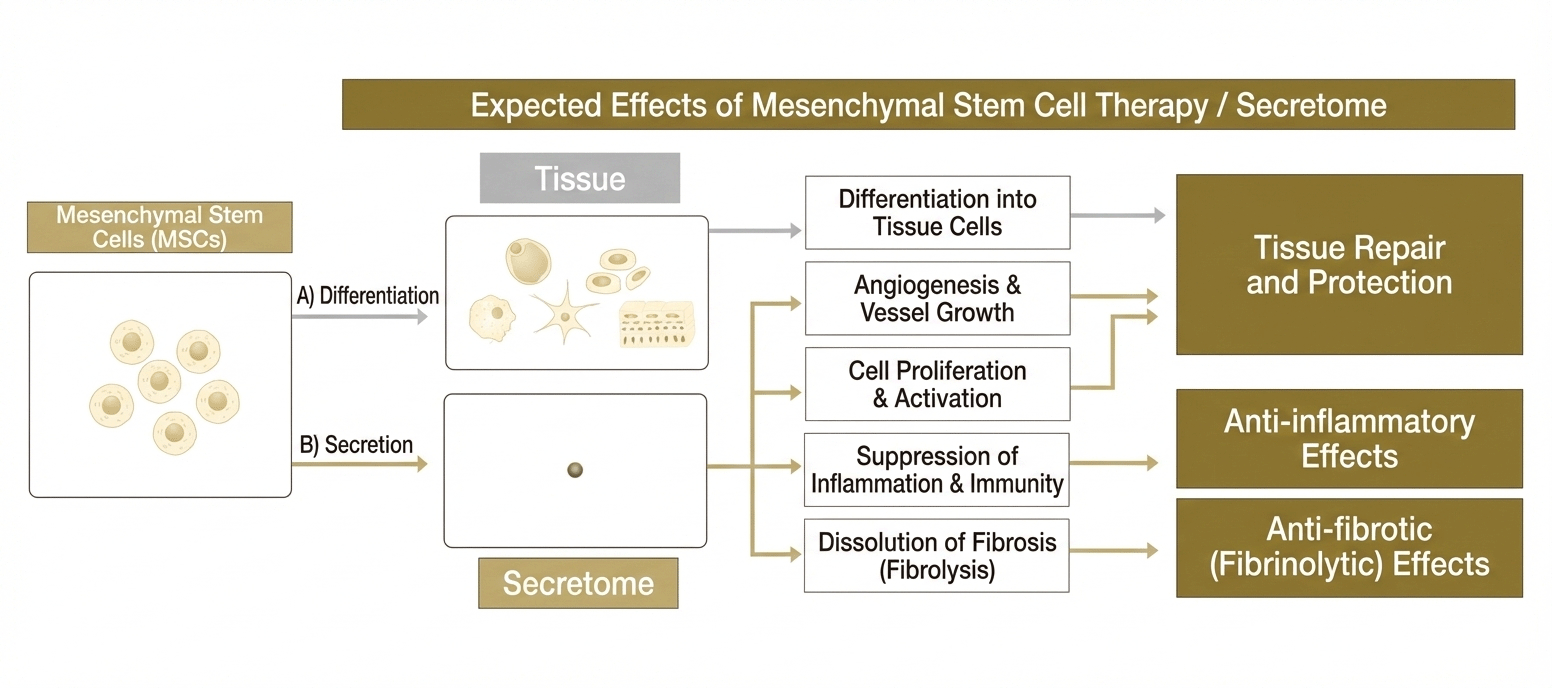
Various effects are expected from secreted factors (stem cell secretome) released by human mesenchymal stem cells.
Autologous stem cell therapy has shown benefits for a wide range of diseases, but manufacturing and quality management are complex, costs are very high, and access is limited to a small number of affluent patients.
With Tele Bio’s advanced proprietary technology—developed by Professor Kotaro Yoshimura, a world authority in this field—manufacturing high-quality, stable stem cell secretome has been made easier and more affordable. The stem cell secretome may offer effects comparable to autologous stem cell therapy.

